Malassezia pachydermatis is a yeast fungus that belongs to the normal microflora of the skin and ear. Its role as an independent primary causative agent of skin disease remains highly controversial. Malassezia is also often present as a saprophyte in the nose, interdigital area, and on the lips, rectum, and vagina of cats and dogs. It has been established that there is a symbiotic relationship between Malassezia and staphylococci, therefore, when cultured from affected skin, they are often isolated together.
Since Malassezia is always present on healthy skin, the clinical manifestation of dermatitis associated with these microorganisms is due to changes in the physicochemical composition of the skin and numerous predisposing factors.
Excessive production of sebaceous secretions, increased humidity and disruption of the epidermal barrier promote the growth of yeast microflora. Predisposing factors may include frequent bathing, allergic diseases, seborrhea, skin damage, long-term use of corticosteroids and antibiotics, internal diseases (especially those affecting fat metabolism), endocrine diseases and more.
There may be a genetic basis for the predisposition of some breeds to Malassez dermatitis (West Highland White Terrier, Basset Hound, Spaniel, German Shepherd, Chihuahua, Poodle, Lha Apso, Dachshund).
Malassezia is also often involved in the development of inflammation in skin fold dermatitis.
Some studies have shown that atopic dogs have an IgE-mediated type 1 hypersensitive reaction to Malassezia.
Allergy to yeast of the genus Malassezia in patients with atopic dermatitis
Lipophilic yeasts of the genus Malassezia, which live on human skin, have unique properties that particularly distinguish them from the kingdom of fungi. The uniqueness lies in the fact that they represent a striking example of the ability of a microorganism, depending on environmental conditions and the host’s immunity, to exhibit the properties of a commensal or a pathogen. In addition, this is the only representative of the human microflora that requires fats for its vital functions. None of the other types of mushrooms have the quality of obligate lipophilicity.
History of the issue. Almost every person's skin, especially the upper part of the body, is colonized by fungi of the genus Malassezia. It would seem that this is why the study of the physiology of this constant companion of the human body should be comprehensive. Meanwhile, the evolution of studying the characteristics of cohabitation between host and fungus for more than a century was accompanied by many errors and inaccuracies. They were first described in 1846 by the microbiologist Eichstedt [1]. After this, for a long time the study of the physiological properties of these fungi was difficult due to the fact that it was not possible to cultivate them. These yeasts did not survive on media that were usually used for cultures of other fungi. It was only when Dr. Benham realized in 1939 that fats were necessary for the life of these fungi that it became possible to obtain a culture of Malassezia spp. [2]. The difficulty of identifying species was also due to the fact that fungi of the genus Malassezia are characterized by dimorphism, that is, the ability to remain in both the yeast and mycelial phases. Therefore, many researchers assumed that these phases are nothing more than different representatives of the yeast flora: the genus Pityrosporum is the yeast form, and Malassezia is the mycelial form. In addition, the variability in the shape of yeast was taken by microbiologists to be different species: the round shape of the cell is Pityrosporum orbiculare, and the oval shape is Pityrosporum ovale. Thus, the taxonomy and nomenclature of the genus Malassezia has been confusing and chaotic until recently [3, 4]. Due to the fact that the presence of two phases of yeast life was never proven, both genera were present in the classification. This situation became clear only in 1977, when three independent groups of mycologists published data that they were able to create conditions under which yeast cells produced hyphae in vitro. Using different variations in culture conditions, they were able to obtain hyphae that were different from the strains isolated from patients with lichen versicolor. Hyphae have been shown to be capable of producing both round and oval shapes of yeast cells, so it has been suggested that cell shape is a phase of the microorganism's life cycle.
Thus, progress in the knowledge of fungi of the genus Malassezia only by 1996 allowed the authors Guillot and Gueho to establish order in taxonomy [5]. They recorded 104 strains of Malassezia spp., identified by DNA signature determined by polymerase chain reaction methods. Based on these data, they identified and named 7 species of the genus Malassezia: M. furfur, M. sympodialis, M. obtusa, M. globosa, M. restricta, M. slooffiae and M. pachydermatis [6].
Biology, physiology, ecology of Malassezia spp. In connection with the problems of cultivation, biology, physiology, ecology of Malassezia spp. poorly researched. The main distinguishing feature of this yeast is its inability to ferment sugars. The main source of carbon for them is fats. Although the microorganism can be grown under aerobic conditions, it can also survive under anaerobic conditions. It is assumed that fatty acids are not a source of energy and do not participate in metabolism, but are an integral part of the cell. The lipids found inside the epidermal cells have the composition necessary to nourish yeast cells [7]. Until recently, the difficulty of obtaining cultural extracts did not allow studies of IgE-mediated reactivity in vivo and in vitro. And only in the last 20 years has it become possible to determine the role of the fungi Malassezia spp. in the pathogenesis of atopic dermatitis (AD).
Antigenic composition of Malassezia spp. The rich antigenic structure of lipophilic fungi is credited with the property of high immunogenic activity, which significantly exceeds that of other representatives of the yeast flora, such as, for example, Candida albicans. The ability of an antigen to cause the induction of antibodies in the human body has an extremely wide polymorphism. It depends both on the state of the host’s immune system, on the antigenic composition of fungi, and on the characteristics of the human environment (climate, composition of water, detergents, insolation, temperature, qualitative composition of the sebaceous secretion of the skin glands). Currently, most of the known antigens of the main species of the genus Malassezia have been described, their biochemical structure, the ability to induce IgE antibodies in the blood of patients with atopic dermatitis have been studied, and 13 main recombinant allergens have been obtained [8, 9].
A special type of immune response, different from that of non-allergic individuals, to Malassezia spp. antigens. is formed throughout life in patients with AD. One of the most significant allergens for sensitizing the body of a patient with AD is mannan of lipophilic fungi, which causes the induction of IgE antibodies specific to it and a positive prick test in half of the patients sensitized to Malassezia spp. Numerous studies have shown that in patients suffering from AD, but not rhinitis or asthma, IgE antibodies to fungi of the genus Malassezia are induced [10]. Several basic biochemical studies have shown high cross-reactivity between antigens of Malassezia spp. and other yeast-like fungi such as Candida albicans, Rhodotorula rubra, Cryptococcus albidus and Saccharomyces spp. It is due to the homology of antigenic determinants of high molecular weight mannoproteins isolated from these fungi. It is believed that primary sensitization is caused by the fungi Malassezia spp. [eleven].
The release of allergens on the skin is influenced primarily by the pH on the skin surface, which is higher in patients with AD than in healthy people. It turned out that M. sympodialis cells produce, express and release allergens in large quantities at the highest pH values. This is especially true for the 67-kDa major allergen, designated Mala s 12 [12].
Immune response to Malassezia spp . There are two conflicting points of view on the phenomenon of induction of IgE antibodies to yeast antigens, due to the proven fact that the levels of specific IgE to Malassezia correlate with the level of total immunoglobulin E, but do not depend on the severity of AD. A number of authors believe that the induction of lipophilic antibodies to mannan is only a manifestation of atopic status, since 85% of patients with AD have a significantly increased level of total IgE, and is not evidence of the role of these microorganisms in the pathogenesis of AD. Others, on the contrary, believe that this is the most important trigger factor for blood pressure.
It is assumed that autoreactivity to human proteins in patients with AD plays the role of one of the most significant pathogenetic factors in AD. One of these proteins, the enzyme manganese superoxide dismutase (SDM) - MnSOD, is induced under stressful situations by both human cells and fungal cells. It has been proven that the enzyme has the property of allergenic activity (autoallergen in AD). Molecular mimicry leading to cross-reactivity, such as sensitization, to MnSOD may be primarily due to colonization of the skin of patients with lipophilic fungi [13].
Thus, lipophilic yeasts of the genus Malassezia, which constantly live on human skin, begin to play the role of a strong antigenic stimulus in patients with AD. If the colonization of the skin surface by fungi occurs in the first years of a person’s life, then in the presence of an atopic predisposition, it is one of the first allergens that causes an immediate type of allergic reaction. Considering the cross-reactivity with other yeast-like fungi, which are constantly present on the mucous membranes, ingested every day with food, with identical enzymes produced by skin keratinocytes, lipophil producers become the main trigger and pathogenetic factors of AD throughout life.
Diagnostics. To diagnose AD complicated by skin colonization with Malassezia spp., mycological, immunochemical, and allergological methods are used.
Mycological research for the purpose of isolating and identifying lipophilic yeasts of the genus Malassezia is carried out by scraping and collecting from the surface of the skin in an area of 1 cm2 on a cotton swab. Next, subculture is carried out in triplicate on Notman-agar (LNA) selective medium: (10.0 g polypeptone, 5.0 g glucose, 0.1 g yeast extract, 8.0 g bovine bile, 1.0 mg glycerol, 0 .5 g glycerol stearate, 0.5 mg Tween 60, 10 ml milk and 12.0 g agar per liter). The inoculated dishes are incubated in a thermostat at 32 °C for two weeks. Species identification of representatives of the genus Malassezia is carried out according to morphological (colony morphology, cell size and shape), physiological (growth at 37 °C, 40 °C) and chemotaxonomic (catalase reaction, utilization of Tween 20, Tween 40, Tween 60, Tween 80) characteristics . The calculation of average CFU/cm2 values is carried out in accordance with the number of colonies grown on a plate from a sample taken from a 1 cm2 area of skin.
According to our data, all patients suffering from AD that we examined, including children, as well as approximately 70% of healthy patients without skin diseases, secrete yeast-like lipophilic fungi of the genus Malassezia. The number of yeasts of the genus Malassezia ranges from 10–106 CFU/cm2. The abundance of yeast isolated from the skin of the upper body is typically one to three orders of magnitude higher than that in samples obtained from healthy patients. On average, the number of Malassezia was 104–105 CFU/cm2 in patients and 102 CFU/cm2 in healthy people.
We were able to identify four species of the genus Malassezia: Malassezia sympodialis, Malassezia globosa, Malassezia furfur, Malassezia restricta. The dominant species in all studied samples was Malassezia sympodialis. We regularly isolated it from the skin of both sick and healthy people. The second place in occurrence is occupied by Malassezia globosa and Malassezia furfur.
Detection of IgE antibodies to Malassezia spp. in the blood serum of patients, it is recommended to carry out the immunochemiluminescence method on the UniCAP 100 device (Phadia, Sweden) using appropriate reagents. We detected IgE antibodies to Malassezia spp. in almost 40% of patients and in none of the healthy patients ( Fig.).
The average values of IgE-AT concentration in blood serum varied from 0.36 kU/l (class 1) to 68.8 kU/l (class 5). They were most often detected in patients older than 7 years; in three patients aged 5 years they were already present in significant concentrations (more than 0.36 kU/l). However, the concentration of IgE antibodies in the group of children was significantly lower (from 0.36 kU/l to 32.0 kU/l, average value 8.06 ± 7.9 kU/l) than in the group of adults (from 0.39 kU/l to 68.8 kU/l, average value 19.42 ± 16.7 kU/l) (p < 0.05).
Thus, although in our study IgE antibodies to Malassezia spp. were detected in only 40% of patients, clinical signs of sensitization to yeast-like fungi were noted in all patients included in the trial according to the relevant criteria. The presence and concentration of antibodies did not depend on the severity of AD. It has been previously shown that in atopic patients suffering from AD, but not rhinitis or asthma, IgE antibodies to Malassezia fungi are induced, so most researchers believe that this is the most important trigger factor for AD.
Thus, the presence of IgE antibodies to fungi of the genus Malassezia in the blood serum of patients with AD is not a sign that determines the severity of the disease, but is a marker of the chronic course of AD. It is very unlikely that during the “atopic march” the appearance of IgE antibodies to fungi of the genus Malassezia in the blood of children will accompany the transformation of AD into rhinitis or asthma with complete remission of dermatitis. The type and abundance of Malassezia yeast on the skin does not affect sensitization to these fungi, that is, there is no dose-dependent effect between the level of antibodies and the degree of fungal colonization on the skin. The data obtained show that the species and quantitative composition of lipophilic fungi living on the skin does not affect the pathogenetic mechanism of AD.
Treatment of AD with skin colonization by Malassezia spp.
Characteristic features of the clinical picture of AD in patients with skin lesions of the upper body are the connection of exacerbations with respiratory infections, the use of foods containing yeast-like fungi and easily digestible carbohydrate foods in the diet, white dermographism of the skin, low therapeutic effect of antihistamines and topical corticosteroids, severe course diseases. The majority of our patients (63%) associated the exacerbation of the disease with eating foods containing sucrose, starch, and flour after more than 24 hours. Nine adult patients noted that when drinking alcoholic beverages, mainly wine and champagne, they experienced severe flushing and itching of the facial skin for 2–5 hours. In all children, the disease worsened significantly after eating food containing yeast (bread, baked goods) and sugar for 24 hours. In this regard, the most significant in the treatment of patients sensitized to yeast is following a diet with a reduced content of easily digestible carbohydrates, foods that promote fermentation, and, most importantly, yeast-containing ingredients. These include, first of all, flour products containing baker's yeast Saccharomyces spp., beer, kvass, champagne, and wine. Yeast-like fungi, such as Rhodotorula spp., are found in almost all canned juices, canned vegetables, and seafood. Increased sugar content in the gastrointestinal tract promotes fermentation and the proliferation of yeast of the genus Candida.
Successful attempts at controlled clinical trials of systemic antifungal drugs in the treatment of patients with AD leave no doubt that the pathology of the immune response of atopic patients to fungi, commensals of human skin, is one of the most significant factors in the pathogenesis of AD. That is, reducing the concentration of antigenic yeast molecules in the body that can cause the induction of IgE antibodies leads to a very noticeable therapeutic effect. But the question of tactics for managing patients with AD remains open. Elimination of yeast from the body is not the final goal of treatment, since in the absence of resident skin flora the risk of infection by pathogenic microorganisms increases significantly. The true solution to the problem is to find that “golden balance” in which, even taking into account the induction of IgE antibodies to yeast, no inflammation of the skin is observed, that is, a symbiotic balance is achieved. It is advisable to prescribe antimycotics (Orungal, Diflucan) only in short courses, during exacerbations, in order to short-term reduce the allergenic exposure to the atopic organism. There are many studies in which the selection of doses and duration of courses of treatment with systemic antifungal drugs was carried out. It is suggested to administer fungicidal drugs in low doses over a long period of more than 6 months. So, for example, Orgungal is prescribed 200 mg per day in 1-2 doses for 2 weeks. If there is no positive effect after 2 weeks, treatment should be continued for another 2 weeks. However, side effects from long-term use of systemic antimycotics are a serious problem that cannot be ignored when prescribing such a prolonged course. That is why, despite successful attempts at treatment with fungicides, these drugs have not yet been included in the standard treatment of atopic dermatitis. And there is no consensus on this issue in the literature yet [14, 15].
The use of local ointment preparations with an antimycotic effect, such as terbinafine, clotrimazole, zinc pyrithione and others, gives a less pronounced but still positive therapeutic effect. Their inclusion in the treatment of blood pressure is undoubtedly an important component, since these drugs do not have the systemic toxicity and withdrawal syndrome characteristic of topical steroids. In addition, we should not forget that daily body care should include cosmetics for the care of the scalp and shower gels with mild fungicidal activity (Nizoral shampoos, Friederm tar, Skin-cap), which patients should use regularly to reduce the number of lipophilic yeasts on the skin.
Conclusion
Recently, questions about the polymorphism of genetic and sensitizing factors in the immunopathogenesis of AD are increasingly discussed in the periodical literature. The absence of IgE-mediated reactivity in 20% of patients with AD with completely similar clinical symptoms suggests a combination of complex reactions, including an inadequate immune response to skin commensals such as Malassezia spp. and Staphylococcus aureus. The unique properties of lipophilic Malassezia fungi allow them to be present on the skin of the human body throughout life almost unhindered. Their protective role as an antibacterial and antifungicidal agent, as an ultraviolet filter and tumor depressant can hardly be overestimated. The striking property of a suppressive and stimulating effect on the immune system makes it possible to experience various polymorphic unfavorable environmental conditions and the condition of the person himself. Skin lesions of the face, neck and shoulders are a characteristic sign of hypersensitivity to Malassezia spp., which is observed in approximately 30-40% of all patients with AD. Thus, sensitization to fungi of the genus Malassezia is one of the most significant trigger factors in the pathogenesis of AD.
Literature
- Eichstedt E. Pilzbildung in der Pityriasis versicolor. Frorip Neue Notizen aus dem Gebeite der Naturkunde Heilkinde. 1846, no. 39, p. 270.
- Benham RW The cultural characteristics of Pityrosporum ovale–a lipophilic fungus // J. Investig. Dermatol. 1939, no. 2, p. 187–203.
- Panja G. The Malassezia of the skin, their cultivation, morphology and species. Trans. 7th Congr. Far East. Assoc. Trop. Med. 1927, no. 2, p. 442–456.
- Slooff WC Pityrosporum sabouraud. In J. Lodder (ed.): “The yeasts—a taxonomic study,” 2nd ed. North Holland Publishing Co., Amsterdam, The Netherlands. 1970, p. 1167–1186.
- Guillot J., Gueho E. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie Leeuwenhoek. 1995, no. 67, p. 297–314.
- Ashbee HR Update on the genus Malassezia // Med Mycol. 2007. V. 45, No. 4, p. 287–303.
- Wilde PF, Stewart PS A study of the fatty acid metabolism of the yeast Pityrosporum ovale // Biochem. J. 1968, no. 108, p. 225–231.
- Koyama T., Kanbe T., Ishiguro A. et al. Antigenic components of Malassezia species for immunoglobulin E antibodies in sera of patients with atopic dermatitis // J Dermatol Sci. 2001, v. 26, no. 3, p. 201–208.
- Johansson S., Karlstrom K. IgE binding components in Pityrosporum orbiculare identified by an immunoblotting technique // Acta Dermato-Venereol. 1991, v. 71, p. 11–16.
- Zargari A., Midgley G., Back O. et al. IgE-reactivity to seven Malassezia species // Allergy. 2003, v. 58, no. 4, p. 306–311.
- Huang X., Johansson S.G.O., Zargari A. et al. Allergen cross reactivity between Pityrosporum orbiculare and Candida albicans // Allergy, 1995, v. 50, p. 648–656.
- Selander C., Zargari A., Mollby R. et al. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens // Allergy. 2006, v. 61, no. 8, p. 1002–1008.
- Schmid-Grendelmeier P., Flickiger S., Disch R. et al. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis // J. Allergy Clin Immunol. 2005, v. 115, 5, p. 1068–1075.
- Back O., Scheynius A., Johansson SGO Ketoconazole in atopic dermatitis: therapeutic response is correlated with decrease in serum IgE. Arch. Dermatol. Res. 1995, v. 287, p. 448–451.
- Clemmensen OJ, Hjorth N. Treatment of dermatitis of the head and neck with ketoconazole in patients with type I sensitivity to Pityrosporum orbiculare. Semin. Dermatol. 1983, no. 2, p. 26–29.
M. A. Mokronosova, Doctor of Medical Sciences, Professor of the I. I. Mechnikov Research Institute of Internal Medicine, Russian Academy of Medical Sciences, Moscow
Why does the disease develop?
Malossezia does not cause skin diseases unless certain conditions are created that promote its development. The conditions that favor the transition of a fungus from a normal microorganism to a pathogenic state have not been sufficiently studied by scientists.
Did you know? Dogs can sense how much time has passed. They may react differently when their owners are away from home for different periods of time.
Most likely, this is facilitated by various kinds of disorders of the body, which normally control microbial colonization. Such disorders may be physical, chemical or immunological in nature. For Malassezia dermatitis to occur, predisposing factors must be present.
Decreased immunity
Immunodeficiency is a possible cause of the disease. Immunoprotective mechanisms normally limit the excessive colonization of the skin by microbes. But if they are violated, the pathogenic development of the fungus increases.
Improper care and living conditions
Frequent bathing of the animal increases the moisture content of its skin. This promotes the development of fungus. Improper care of animals with skin folds or floppy ears can also lead to its development. Malossezia develops on skin whose pH is in the range of 4–8.
The skin of dogs has the highest pH among mammals. This figure may vary depending on the breed. More alkaline skin is more susceptible to infections than acidic skin. High pH levels in dog shampoos can cause irritation if there are skin problems.
Chronic diseases
A number of diseases lead to the development of fungus: food allergies, endocrine diseases, dermatitis, keratinization, viral leukemia. The relationship between various diseases, infections and fungal growth is not well understood. But treating the disease can sometimes prevent its development or reduce its manifestation.
Important! If an increased proliferation of staphylococci is observed on the skin of an animal, this provokes the development of yeast fungi, since the species are in a symbiotic relationship.
Other reasons
The prevalence of the disease is influenced by the climate of the area. High ambient temperatures with high humidity favorably affect the growth of the fungus, while dry weather conditions prevent this process. An increase in ambient temperature in summer and spring leads to increased sweating, which can trigger the development of the disease.
What dog breeds are predisposed to Malassezia?
There is no predisposition of an animal to Malossezia depending on age and gender. But there are breeds in which the disease appears more often. Among them it is worth highlighting: basset hound, Scottish and Australian terriers, cocker spaniel, dachshund, miniature poodle, shih tzu.
How do symptoms appear?
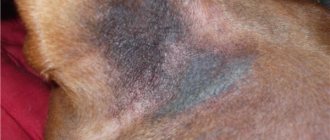
Skin lesions can be observed in one place or scattered. They are mainly found on the animal's face, the front of the neck, in the external auditory canal, in the axillary fossae, groin area, interdigital folds or in areas with diaper rash. Symptoms of the disease include redness of the skin, partial or complete baldness in the affected areas, scaliness or excessive fatty discharge.
Important! The fungus Malassezia pachydermatis is not transmitted to humans.
In chronic cases, thickening of the skin is observed as a secondary element of the rash, increased skin pattern, and impaired pigmentation of the affected area. Itching of the skin can be of varying degrees. The disease is distinguished by a specific skin odor and brown discharge from the ear canal. The animal's claws also turn brown.
Alternatives to steroids in the treatment of atopy
Most owners are referred to a veterinary dermatologist when steroids are no longer effective or an underlying medical condition prevents their use (eg, diabetes, heart disease). Since house dust mite allergy appears to be a common allergen that exists year-round, steroids are not an option.
In seasonally affected animals, occasional steroid injections or oral prednisone may be helpful. Long-term use should be avoided as there are other options.
Although not intended for use in cats, cyclosporine has become a good alternative to steroids in the treatment of atopic cats. The drug has been used since the 1970s for kidney transplants, even at higher doses than in dogs. (7.5-10 mg per kg per day).
For cats with atopic dermatitis, I recommend using 5 mg/kg or even lower to start. I start with a low dose to avoid the gastrointestinal side effects of vomiting or diarrhea. Either cyclosporine capsules or cyclosporine modified oral liquid 100 mg/ml are used. And we start with 0.1 ml.
The disadvantage of oral liquid is that the 50 ml bottle must be used within 60 days. The advantage, in my opinion, is less gastrointestinal upset. Be sure to check for toxoplasmosis status before use. Use of cyclosporine may cause relapse of the disease.
It may be wise to avoid cyclosporine in outdoor cats given the possibility that they are eating raw meat. Some have been observed to develop mild neutropenia. Therefore, it is important to check a complete blood count and serum profile before and regularly during use.
After success in taking cyclosporine daily, switch to taking it every other day or even less. It has the advantage that it can be administered orally and does not require several months to be effective, unlike immunotherapy.
Atopic dermatitis in cats is a reality and difficult to treat, let alone diagnose.
Luckily, there are more options than just steroid injections, which were previously considered safe for felines.
We now know that diabetes, demodicosis and heart disease are sometimes associated with hormone use. Since atopy is lifelong, it is preferable to use the alternatives available to them, which are much safer.
To receive detailed advice or qualified assistance, contact me for help in any way presented on the site. More information on the page: treatment of skin diseases in cats
Therapy
To treat Malassezia in cats, external antifungal drugs are used, which include:
- shampoos;
- ointments;
- solutions.
To make the therapy more effective, it is better to cut off the long hair or shave your pet before applying these products.
In addition to this treatment, tablets should be given orally that suppress the development of fungal microflora in the body. Only a doctor should decide the advisability of using them in kittens, since they have a detrimental effect on liver cells.
The cat should be washed with shampoo containing fungicides once every 3 days. The procedure must be carried out over three weeks.
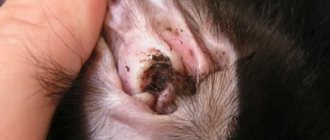
For Malassezia otitis it is necessary:
- Clean the ears from accumulated exudate 2 times a day;
- treat the ear canal with an antiseptic;
- instill antifungal drops;
- apply oral fungicides.
If there are concomitant infections, treatment is carried out with antibiotics.
Food allergies can be eliminated by changing your pet's diet.
It should be understood that a timely diagnosis and correctly prescribed complex therapy can give a quick positive result.
Ear hematoma in cats
With a hematoma, blood flows from the blood vessels of the auricle into the tissue under significant pressure, pushing these tissues apart and forming a cavity. The size of the hematoma depends on the strength of blood pressure in the damaged vessel, as well as on the degree of compliance of the tissues located near it.
A hematoma occurs quickly and its volume increases until the pressure from the stretched tissue becomes equal to the pressure in the damaged blood vessel. After this, the spilled blood clots, and a blood clot forms in the blood vessel.
Most often, hematomas in cats occur on the inner surface of the ear and much less often on the outside. The damaged ear increases in size, hangs down, and the swelling is painful and hot to the touch. If the hematoma is not treated, the pain only increases, and the hematoma itself can become infected with secondary microflora, which can ultimately lead to necrosis of the ear cartilage.
During a clinical examination of such a cat, a veterinarian notes the following symptoms:
- We observe anxiety and nervousness in the cat.
- The cat almost constantly shakes its head from side to side.
- He constantly scratches his damaged ear with his paws.
- When trying to stroke the cat's head, it becomes aggressive.
Treatment. Treatment of auricular hematoma is not very difficult. If no more than 48 hours have passed since the formation of the ear hematoma, then the cat owner fixes the ears with a bandage on the back of the head and applies cold. In the future, in order to resolve the hematoma, it is necessary to use heat and apply locally irritating ointments.
If the hematoma cannot be cured at home, the owner needs to contact the nearest veterinary clinic. In a veterinary clinic, the veterinarian will open the resulting hematoma, remove blood clots from it, wash the resulting cavity with a solution of novocaine with antibiotics and give recommendations to the owner so that the hematoma resolves safely.
Is vaccinating cats against lichen effective?
Today, only Russian vaccines for the prevention of lichen in animals are available in veterinary pharmacies. Abroad, such remedies have disappeared and are not used due to the low prevalence of the disease.
Since in Russia lichen is very often registered in agriculture, fur farming and among domestic pets, there is a demand for preventive biological products. However, to date, no pharmaceutical company has published reliable data or studies that support the effectiveness of vaccinating animals against zoster.
Science has not proven that antifungal vaccines can protect against possible infection. Even if you repeatedly vaccinate kittens or rabbits against lichen, upon contact with the pathogen, the animals still get sick and go through all stages of clinical development on a par with those who were not vaccinated.
The effectiveness of the vaccine was studied on a small group of experimental animals, the result was positive, so a vaccine against lichen exists.
In fact, biological products (such as vaccines) require larger studies and many animals with lichen (and pets are selected based on similar physiological parameters and the same stage of development of the disease), which is extremely difficult and financially expensive. Therefore, many experienced veterinary specialists are convinced that vaccinations against lichen are not a preventive measure. However, vaccine manufacturers have their own point of view on this matter.
How is the diagnosis carried out?
To identify the fungus Malassezia pachydermatis, various diagnostic methods are used. These include cytological, pathological tests and culture. Cytological examination makes it possible to quickly determine the presence of the fungus. To do this, transparent tape is glued to the affected skin and then removed. The sample taken is subject to special staining and examined under a microscope with a magnification power of one hundred times. If the fungus is easily established, it is concluded that the skin is infected. In case of single detections, the analysis is considered to have given a negative result. Dry scrapings and smears are alternative cytological methods.
To quantify the fungus, a method using Petri dishes is used for diagnosis. The fungus is cultivated in small dishes with a special propagation medium, applying it to the affected area for 11 seconds. For 4–7 days at a temperature of 32–37°C, conditions are created for growing the mushroom. The result indicates the density of the fungal population. It is interpreted taking into account the location of the infected area on the animal’s body and the breed of the dog.
Important! Using a biopsy, you can see the fungus in the dry affected layer of skin. Histological sections do not always give reliable results.
Is it possible to develop immunity to fungus?
Cats do not develop resistance to fungal forms of lichen after an illness. General immunity is produced exclusively by blood cells when a foreign agent penetrates the bloodstream.
Lichen fungi do not penetrate blood vessels and live on the surface of the skin and in the animal’s fur. Accordingly, a cat that has once had shingles can become infected again.
There is a theory about the presence of local (skin) immunity in the body of living beings. That is, skin leukocytes recognize antigens (in this case, fungi) and produce antibodies to them. Moreover, lichen fungi come into contact with the surface of the skin. It is on this fact that the assumption about the effectiveness of vaccinations against lichen is based.
How contagious is the fungus in Malassezia?
When doctors use the term “malassezia,” they mean a fungal organism of the yeast species. It is slightly convex in shape and has a cylindrical structure, smooth, soft, loose, and small (if translated into microns, it is two and a half microns). Looking at it, you may think that you are seeing an ordinary nut. Malassezia crops are characterized by an unusual smell with a “fruity” tint.
When you see someone with Malassezia dermatitis, you don’t have to run away in fear or hide your pet away. The idea that Malassezia fungus is contagious is completely false. Since it is one of the components of the microflora, which tends to be present on the epithelial tissue of the animal throughout its life.
In everyday life, the fungus causes absolutely no harm. Excessively fast and large reproduction begins after any incidents that create an excellent environment for this. Most often this is provoked by various changes in the microclimate of the skin (humidity, warmth, advanced inflammation).