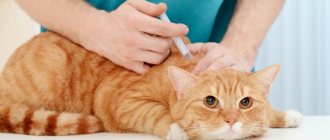
Vaccination is an important procedure that is necessary to protect the health of an animal from various infectious diseases. But even if your pet gets sick, he will survive the illness much easier. For these purposes, medical immunobiological drugs are used, among which Nobivak is the most popular. However, like any medicine, it has its own indications, dosage, and contraindications. Therefore, knowing how to use Nobivak for cats, you can prevent the development of serious diseases. More about this in more detail.
Varieties
Vaccinations are necessary not only for pets that walk outside, but also for pets. The Dutch manufacturer's line includes several series, divided depending on the type of mammal and the infections against which they are intended.
Nobivak Tricat Trio
Produced since 1999 and protects the body from three infections at once:
- calicivirus is a fairly common disease that mainly affects the respiratory system. The greatest danger is for kittens, weakened and elderly individuals. Often leads to death;
- When rhinotracheitis becomes infected, the respiratory organs and eyes are affected. The lethal outcome reaches 10-15% of the total number of infected;
- panleukopenia or feline distemper, which is highly contagious. It is characterized by a feverish state, dehydration, damage to the digestive tract, respiratory and cardiovascular systems. The causative agent is parvovirus, the mortality rate of kittens is up to 90%. If an adult animal has survived the first 3-4 days of the development of the disease, then the chances of recovery increase, but in any case, it will remain a lifelong carrier of the virus.
Protection against three infections
One dose of the product is available in 2 bottles:
- a live vaccine, created from cells that are infected with 3 viruses with the addition of stabilizing components, presented as a pink or yellow powder;
- Diluent solvent (1 ml), represented by a mixture of water for injection and additional components.
Nobivac Rabies
Rabies is indicated for the prevention of rabies, a dangerous disease that causes inflammation of the brain and spinal cord with damage to the central nervous system. It is impossible to save an infected animal; as a rule, they are euthanized, as it poses a risk to people. A person can become infected from a bite from an infected fluffball.
It is hypoallergenic, has a yellow or pink tint, and the presence of easily soluble sediment is allowed. The composition contains dead cells infected with the rabies virus in combination with an adjuvant that increases immunogenicity and glycerin.
Prevention of rabies
Nobivak VV
It is aimed against bordetellosis, which provokes inflammation of the respiratory tract and lungs. The greatest danger is for kittens and older adults. It is not considered vital, but it is recommended when keeping several pets together, as they can easily become infected from each other by sneezing, through saliva, or nasal discharge.
Vaccine against bordetellosis
One dose consists of 2 bottles:
- the vaccine is presented as a white powder, which is made from a weakened strain of pathogenic microbes, with stabilizers;
- solvent 0.5 ml water for injection.
Nobivak Forcat (Forcat)
This remedy is effective against calicivirus, panleukopenia, rhinotracheitis and feline chlamydia, which is a serious disease that provokes inflammation of the mucous membranes of the eyes and respiratory system. Most often observed in kittens at the time of weaning from maternal feeding.
Nobivak
Available in 2 bottles:
- whitish-pink powder with stabilizers;
- solvent Diluet 1 ml.
Nobivak Tricat Trio
This is an immunobiological drug, the manufacturer of which (like other vaccines) is. The dry vaccine contains weakened viruses that, after special preparation, are not capable of mutating or causing disease. It helps to form specific immunity to three dangerous infections.
The basis of the drug is a culture liquid from FEF cells. It is infected with the following viruses: rhinotracheitis (strain G 2620) – 5.2 lg PFU (plaque-forming units), calcivirosis (F9) – 4.6 lg PFU and panleukopenia (MW-1) – 4.3 lg TCD50 (tissue cytopathogenic doses). Gelatin, sorbitol, PGA, disodium hydrogen phosphate dihydrate and injection liquid are used as auxiliary components.
The drug is sold together with a solvent that contains disodium hydrogen phosphate dihydrate, potassium dihydrogen orthophosphate and injection water.
Preparing your pet for vaccination
An absolutely healthy animal must be vaccinated. 10-14 days before the procedure, you need to visit a veterinarian, take the necessary tests, and also carry out anthelmintic therapy for the purpose of prevention, and if any, until complete destruction. If a family has several pets, then they all need to be vaccinated at the same time.
When is vaccination prohibited?
If any health problems are detected (cold symptoms, lethargy, intestinal upset, hyperthermia, inflammatory processes), immunization is postponed until complete recovery and can only be carried out after examination by a veterinarian.
At an appointment with a veterinarian
Also, vaccination is not carried out if:
- Less than 14 days have passed since taking antibiotic therapy;
- surgical intervention is planned, then immunization is carried out no later than 3 weeks before and 3 weeks after surgery;
- at least 8 weeks old;
- there is pregnancy or lactation period.
special instructions
When performing procedures using the drug "Nobivak", some caution should be taken so as not to harm the cat's body. Be sure to adhere to the rules of asepsis to prevent infection and complications. It is also important to pay attention to other rules:
- If the solution gets on the mucous membrane of the visual organs, you need to rinse them with plenty of running water.
- After the manipulation, the person needs to wash their hands with soap.
- If, through negligence, the medicine “Nobivak” was introduced into the human body, then you need to contact a medical facility as soon as possible.
How to use the vaccine
Each vaccination is done according to a specific scheme. Vaccination with BB is carried out according to the following algorithm:
- Add a solvent to the powder and shake well;
- warm in your hand for about 60 seconds;
- the solution is drawn into a syringe, while replacing the needle with a nozzle for injection into the nasal passages;
- The pet’s head is raised, the mouth is clamped, and an injection is made into the nose.
1 bottle contains 1 dose. Prescribed from 4 weeks, revaccination at 1 year and then annually.
For Triket and Forket there is a slightly different method:
- the powder is dissolved with a solvent until smooth;
- the injection site is treated with an alcohol solution;
- The medicine is drawn into a syringe and injected under the skin.
Prescribed from 8-9 weeks, re-vaccination after 3-4 weeks, revaccination annually, then annually.
Prick at the withers
The rabies vaccination is presented as a suspension and therefore does not require special preparation. The ampoule is shaken, drawn into a syringe, and injected subcutaneously. Simultaneous administration with Triket and Forket is possible, but in different syringes and in different places. Carry out at 8-9 weeks, revaccination at 1 year, then once a year.
Storage conditions
“Nobivak” for cats must be stored in a dark, child-proof, ventilated place at a temperature of 2-8°C (25°C for solvent). It is not recommended to store the medicine together with human or cat food, or in a general first aid kit.
Shelf life : Nobivak Bb and solvent - 5 years, Rabies - 4 years, Trio and Forcat - 2 years and 9 months (35 months), Dukat - 2 years.
If the expiration date has expired, there is external damage, or there is a change in color or consistency of the medicine that is visible to the naked eye, the bottle should be decontaminated and discarded. That is, boil the bottle with the product for 20 minutes and then throw it away. Do the same if the bottle is opened and not used within 30 minutes after that.
Contraindications
Of course, in order to develop stable immunity, the pet must be absolutely healthy. Contraindications to immunization are:
- temperature increase;
- dehydration, exhaustion of the body;
- state of immunodeficiency;
- helminthic infestation;
- recent injuries, serious illnesses, surgery.
According to indications, vaccination is possible during the period of gestation of kittens and feeding them with milk
Advantages and disadvantages
Like any veterinary drug, Nobivak has positive and negative properties, which must be taken into account by owners before carrying out manipulations on cats. The main ones are presented in the table:
| pros | Minuses |
| High performance | High cost, which is why the drug is not available to every pet owner |
| Convenient use | |
| Development of long-term immune protection against many infectious diseases in cats | |
| High quality - made in the Netherlands | |
| Minimal likelihood of adverse reactions |
Does CoviVac protect against omicron?
Studies have not yet been conducted to determine whether CoviVac protects against Omicron, a new strain of the SARS-CoV-2 virus. There is no data on the effectiveness of the Omicron vaccine, but there is information that suggests that the level of protection will decrease as the new strain spreads. It is known to bypass immune defenses better than previous variants of the coronavirus. Because of this, the number of cases of COVID-19 among those who have already had it or have been vaccinated is increasing.
Despite this, in the Center. Chumakov positively evaluates the effectiveness of the CoviVac vaccine and its effect on omicron. It is assumed that even with the mutations of the coronavirus, antibodies formed after vaccination will provide the necessary protection. On the other hand, the effect of the vaccine even against the first “versions” of SARS-CoV-2 has not yet been confirmed by clinical studies, so it is too early to judge whether CoviVac protects against the omicron strain.
Since the beginning of the spread of the omicron strain in the center named after. Chumakov made several statements. In mid-December 2022, the center suggested that the vaccine “should be effective” and did not need to be modified. Later, the period for such a modification was estimated at six months. At the same time, the Center’s management notes that omicron is a temporary “version” of the virus, and therefore the development of a special vaccine that will protect against the new strain is not justified. Also in January 2022, it became known that vaccine developers were preparing for studies that would help evaluate its effect against the new strain.
Omicron is a new strain of coronavirus that is rapidly sweeping the world. It is supposed to be less dangerous than the delta strain. Photo: MrDm / freepik.com
The effectiveness of the CoviVac vaccine in Omicron
It is not yet known whether CoviVac will help against omicron if a person is already sick. It is assumed that for the new strain the risk of becoming seriously ill may be higher than for previous “versions” of SARS-CoV-2. It is also difficult to assess the protection that CoviVac provides against the omicron strain because this vaccine is not widely distributed. In 2021, its production was suspended for production modifications. It is expected that large deliveries will begin only in 2022.
When assessing the impact of vaccination and the effectiveness of CoviVac against omicron, one must take into account that the presence of antibodies still reduces the risk of severe disease, hospitalization and death. Therefore, it is important to use all preventive measures, including vaccination and compliance with normal precautions.
Vaccine manufacturer
One of the common questions related to CoviVac is who makes the vaccine? The vaccine "CoviVac" is produced by the Federal State Budgetary Institution "FNTsIRIP im. M.P. Chumakova RAS.” Center named after M.P. Chumakova has been working since 1957. This is a large research and production complex, which includes 43 divisions. Center named after M.P. Chumakova is the only manufacturer of live vaccines against polio in Russia, the official supplier of vaccines against yellow fever. The company is also the only supplier to UNICEF and WHO in Russia. This is the leading Russian pharmacological enterprise among commercial vaccine manufacturers².
The production of CoviVac began in March 2022. The drug is already used for vaccination. It is known that phases 1 and 2 of clinical trials have been conducted for it, but there are no publications on them yet, and precise data on tolerability or protective characteristics are unknown. It will be possible to judge the effectiveness and ability of the vaccine to protect against coronavirus infection only after the completion of the third phase of clinical trials. It is expected that 32 thousand people will participate in them. The research should be completed at the end of 2022.
The first and second phases of clinical trials of the CoviVac vaccine began in August 2022. They were conducted to evaluate safety, tolerability and ability to induce an immune response. 600 people aged from 18 to 60 took part in them. Until the results of the studies are published, it is impossible to judge even how safe the drug really is. Moreover, it is impossible to draw conclusions about whether the CoviVac vaccine is capable of protecting against coronavirus - this information will appear only after the publication of the results of the third phase of clinical trials. So far, even preliminary estimates of effectiveness vary greatly.
Preliminary estimates of the effectiveness of the CoviVac vaccine
“The effectiveness is not clear.
There are no publications. According to the results of groups of volunteers, very few antibodies are produced. Thus, the effectiveness will be lower, since antibodies are an indicator of the degree of activation of the immune system, like a light bulb on a motor - whether it works or not. Foreign analogues have not been very successful in civil vaccination. Developers of similar Chinese inactivated vaccines claimed high - more than 80% - effectiveness. However, countries that have vaccinated their populations with these vaccines are faced with a harsher reality. So by June, the Seychelles, Chile, Bahrain and Mongolia had between 50 and 68 percent of the population fully vaccinated, ahead of the United States. All four countries were among the top 10 countries with the worst coronavirus outbreaks in June, all of them mainly using Sinopharm and Sinovac Biotech."
Dudnakova Tatyana Valerievna
expert
Research Fellow at the University of Edinburgh, UK
“Whether the CoviVac vaccine is effective is still unknown. It has the prerequisites to be effective, because there are two Chinese-made vaccines made using similar virus inactivation technology. But until the third phase studies are conducted, we will not know whether the CoviVac vaccine is effective or not. The fact that, according to developers, it causes an immune response in 80% of patients is good, but does not yet guarantee protection. It may well turn out that they inactivated the virus in such a way that the vaccine turned out to be less effective. It’s difficult to imagine this theoretically.”
Yasny Ilya Evgenievich
expert
head of scientific examination of the pharmaceutical investment fund "Inbio Ventures".
The CoviVac vaccine was created on the basis of a producer strain obtained from one of the patients of City Clinical Hospital No. 40 Kommunarka. A sample was selected for development that already had several mutations compared to the original virus that originated in Wuhan. Among these mutations was D614G, a change in the S protein that helps the virus particle attach to and enter cells. The D614G mutation is very common and makes the virus more infectious. A sample with this mutation was cultured in Vero cell lines, after which the AYDAR-1 strain was obtained. It became the basis of the CoviVac vaccine.
During production, the virus is grown in Vero cells. After this, it is killed: the shell is destroyed and the RNA is inactivated, and then purified. As a result, only whole-virion particles and fragments of virus proteins remain, incapable of infecting cells and reproducing.
Production of the CoviVac vaccine at the Federal Scientific Research Institute of Infectious Diseases named after. M. P. Chumakova RAS. Photo: frame from the First Channel story on YouTube
The essence of the CoviVac vaccine
In fact, the CoviVac vaccine contains a “killed” virus that is not capable of harming the body, but can “train” and educate the immune system. The advantage of this approach is that the immune system becomes familiar with the entire virus at once, and not with its individual particles. It is expected that this will help it better recognize SARS-CoV-2 and more effectively resist it³.
What the owners say (Review)
“I have always loved cats, but I was able to have my own pet only when I started living separately from my parents (my mother has allergies). They scared me with various cat diseases. For some reason, I was most afraid of rabies. But she still decided on a new family member, although she rushed straight to the clinic for a full examination and consultation. The doctor vaccinated the kitten with Nobivak and calmed all my fears. Adaptation to the pussy's new home was painless. And the vaccination, apparently, did not bring any consequences. The new tenant happily ran around the apartment, did not lose his appetite, slept well and did not try to touch or lick the injection site. Two weeks later, we applied the same Nobivak, only with a different vaccine, to the nose to exclude several more serious diseases. And this time, too, everything went fine. True, the kitten sneezed so funny for some time. I’ll have a baby soon, but I’m not at all worried that my furry hero will be somehow dangerous for him.” Tatyana, Sverdlovsk.
Nobivac safety
The product containing attenuated strains of pathogens is actively used for administration to cats of all breeds of different ages.
The attractiveness of this modern immunoprophylactic agent is that:
- the pharmaceutical drug causes rapid formation of immunity to the three most dangerous cat diseases;
- characterized by a high level of safety;
- the produced antigens are characterized by long-term activity.
After the introduction of any type of the Nobivak series, complications do not arise. The following fact should be especially emphasized: although vaccinations with this drug are given mainly to small kittens, their body, despite age-related vulnerability, can easily cope with the effects of a harmless drug.
Read. "Havrix": instructions for use, reviews, cost
Consumer Opinion
Reviews from many cat owners coincide with the professional conclusions of veterinarians:
- these are safe and effective remedies for animals against a number of terrible diseases;
- the majority of pets tolerate vaccination very well;
- negative reactions to vaccination are extremely rare and short-lived;
- ease of use allows cat owners to vaccinate them themselves;
- Thanks to affordable prices, drugs are available to the entire population.
Different opinions about the drug
There are many positive reviews about the Nobivak Triket vaccine . The owners say that their pets tolerate the injection well, and the drug itself does not cause any side effects, even in small kittens. This medicine is also very convenient to use due to the fact that it protects the animal from several diseases at the same time. This eliminates the need for other vaccinations. The injection can also be done at home.
Another group of people report that after vaccinations, cats experienced vomiting, weakness, lethargy, and diarrhea. But later it turned out that these animals suffered from intestinal infections, gastritis, and helminthiasis. These diseases were latent. Vaccination in this case aggravated the ailments.
It should be remembered that the animal may have distemper, calcivirosis, or rhinotracheitis. In this case, the drug will most likely lead to serious manifestations of infection.
Such infections can only be diagnosed using PCR. A sick cat may have no symptoms in the initial stages.
Operating principle
The CoviVac vaccine is inactivated. This means that it contains a “killed”, neutralized virus, which, nevertheless, is capable of causing a reaction from the immune system. It is obtained in several stages:
- Growing Vero cells. These are special cell lines that are used to culture the virus.
- Coronavirus infection. The cells become infected with the AYDAR-1 strain of coronavirus.
- Inactivation. The virus undergoes chemical treatment and dies. Once inactivated, it cannot infect cells or reproduce.
- Cleaning. To produce the drug, the antigen is purified so that only the inactivated virus and its protein particles remain.
After injection, the antigen appears in the soft tissues. In this place, the concentration of immune cells quickly increases. They recognize the inactivated virus and begin to fight it. It is assumed that as a result of this, protective antibodies will be formed in the blood, which will be able to destroy the “live” virus in case of contact with it.
Figure 1. Live and inactivated virus. Image: kavusta/Depositphotos
To neutralize the coronavirus, beta-propiolactone is used in the production of the CoviVac vaccine. This is a chemical substance, a toxin, that partially destroys proteins and nucleic acids upon contact with them. CoviVac is not the only inactivated vaccine against coronavirus: such drugs are also being developed in other countries. Almost always, beta-propiolactone is used to inactivate the virus. This toxin destroys nucleic acid, but at the same time changes protein structures less. In the case of coronavirus vaccines, this is important because when infected, the virus first interacts with living cells through the “spike” proteins on its surface, which form a “crown.” With their help, it attaches to cells and penetrates them. To protect against infection, the immune system needs to respond quickly to the spike proteins. It is assumed that inactivation with beta-propiolactone allows one to preserve these proteins in sufficient quantities, but at the same time reliably neutralize the virus.
Is the virus actually killed in the CoviVac vaccine?
“This is truly a killed virus.
The problem is rather the opposite - proteins that are too chemically denatured. After the tragedy in the USA in the 50s, when the polio virus was not completely inactivated, they now pour in a lot of chemicals and do several tests on each batch on cell cultures and animals. So there will be no live virus there. But the covid spike complex has a labile structure, which is disrupted during chemical treatment, and some important epitopes are lost - therefore, few useful antibodies are formed, because it is the antibodies to the spike protein that are needed.” Dudnakova Tatyana Valerievna
expert
Research Fellow at the University of Edinburgh, UK
Inactivation raises many questions regarding the safety and effectiveness of the CoviVac vaccine. The virus must definitely be killed, but it is important not to “spoil” it. After chemical treatment, some of the proteins important for the formation of immunity are destroyed. In the structure of the SARS-CoV-2 coronavirus, the S-proteins (spikes) are loosely connected to the viral particle, and there is a risk that they will simply collapse when inactivated by beta-propiolactone. Employees of the Center named after. M.P. Chumakova argue that these risks have been taken into account, and the virus used is, on the one hand, safe, and on the other, suitable for training the immune system.
Figure 2. Lymphocytes attack coronavirus. Photo: Sakurra / Depositphotos
What other vaccines use inactivated viruses?
There are many such vaccines - they use traditional, “classical” technology, which is well proven. Only in the Center. M.P. Chumakov produces similar vaccines against polio, tick-borne encephalitis, rabies, and yellow fever. Several different inactivated vaccines have already been developed against coronavirus: for example, Sinopharm, CoronaVac, BBIBP-CorV (China) or Covaxin (India). They are all similar to each other. The main difference between them is the choice of strains used. There are also minor differences in the virus inactivation technology.
Is a vaccine with an inactivated virus a thing of the past? Maybe you need to choose more modern options?
“Yes, inactivated vaccines are becoming a thing of the past.
There are many disadvantages. It is dangerous to work with the pathogen itself. During inactivation, denaturation occurs, a violation of the structure of protein complexes, and there are two consequences - some important epitopes are destroyed, that is, protective antibodies are produced worse, and denatured proteins can become similar to internal complexes - autoimmune reactions are possible. The main disadvantages are long development, complex production with scaling problems (it is difficult to select the amount of chemicals, since the vaccine is always developed slightly differently for each virus), and a product with low efficiency. The Argentine Ministry of Health reported on July 1, 2022 that preliminary results from a sample of 471,682 Argentines over 60 years of age in February-June 2021 showed an efficacy against death from COVID-19 of 61.6% for the first dose of Sinopharm's BBIBP-CorV vaccine. and 84.0% for the full two-dose course of vaccination. For comparison: under the same conditions, the AstraZeneca vaccine showed an effectiveness of 79.5% and 88.8%, the Sputnik V vaccine - 74.9% and 93.3%. The future is clearly in vector and mRNA vaccines.” Dudnakova Tatyana Valerievna
expert
Research Fellow at the University of Edinburgh, UK
Instructions for use
The diverse effects of Nobivac strains are united by some general rules for any vaccinations performed:
- Each vaccination is carried out under sterile conditions (hands protected with medical gloves, disposable injection syringe).
- One ampoule with a vaccine strain + 1 bottle of solution is one vaccination (0.5 cm3 of powder per 1 ml of Diluent) or one instillation of IV (0.3 ml).
- The medication is administered subcutaneously or intramuscularly. The only IV drug that is injected into the nasal sinus (with a syringe without a needle).
The pet must also be prepared in a certain way:
- 10 days before vaccination, the animal must carry out preventive measures for deworming (give the cat an anti-parasite drug).
- Nobivak BB can be used once the animal reaches 1 month of age.
- Other types of Nobivac are vaccinated only after 2 months of age.
- If there are several mustachioed pets in the family, all must be vaccinated at the same time.
- The effect of the vaccine lasts for a year. Then a second injection is required.
After vaccination, your pet must be monitored for at least 3 days. This is exactly how long it takes for the virus strains to begin interacting with the circulatory system. It happens that the pussy does not tolerate the vaccine injection well, then it needs to be urgently shown to the doctor.
Sources
- Registration certificate and Instructions for medical use of the medicinal product CoviVac (inactivated whole virion concentrated purified coronavirus vaccine) dated 02/19/2021.
- Ministry of Health of the Russian Federation. Instructions for the medical use of the medicinal product CoviVac (inactivated whole virion coronavirus vaccine, concentrated purified).
- Ministry of Health of the Russian Federation. Temporary guidelines “Procedure for vaccination of the adult population against COVID-19”, July 2, 2022.
- Ministry of Health of the Russian Federation. Instructions for the medical use of the medicinal product “CoviVac” (inactivated whole virion coronavirus vaccine, concentrated purified).
- “Sputnik V”, “EpiVacCorona”, “CoviVac”, “Sputnik Light”. What is known about Russian vaccines. TASS, 2022.
- Ministry of Health of the Russian Federation. Temporary guidelines “Procedure for vaccination of the adult population against COVID-19”, July 2, 2022.
- Expert: the use of different drugs during vaccination and revaccination against COVID-19 is possible. Interfax, 2022.
- The CoviVac vaccine will be modified to accommodate new strains of coronavirus. RIA Novosti, 2022.
- The Russian Ministry of Health has authorized testing of CoviVac on the elderly. Interfax, 2022.